Case Studies
Development of a Stroke Disease Model
Overview
This is an example of the development of a disease progression model for stroke used to evaluate the activity of a new drug candidate. First a pharmacokinetic model was developed for the drug, then a disease progression model was developed for the recovery from ischemic stroke, and then the effect of the new agent on recovery was evaluated. The disease progression model was able to identify the decreasing benefit over time of tPA on stroke recovery as well as a slight benefit from the new agent.
POPULATION PHARMACOKINETIC AND PHARMACODYNAMIC MODEL OF CP-101,606 FOLLOWING A 72-HOUR INFUSION IN ACUTE ISCHEMIC STROKE PATIENTST. TAYLOR M.S.,1 D. Mould, Ph.D.,2 P. Ravva, M.S.,1 M. Krams, M.D.,1 J. Weaver, M.S.,1 T. Morse, B.S.,1 M. Bednar, M.D., Ph.D.,1 L. Benincosa, Ph.D.1Pfizer Global Research & Development, Clinical Research & Development, Groton/New London Laboratories, Pfizer Inc, Groton, CT 06340; 2Projections Research Inc, 535 Springview Lane, Phoenixville, PA 19460
Introduction
CP-101,606, a substituted 4-phenylpiperidine, is an NMDA receptor antagonist that is selective for receptors containing the NR2B subunit. Pathologic overactivation of the N-methyl-D-aspartate (NMDA) class of receptors has been implicated in the neurodegeneration that occurs following stroke.1 There are multiple subtypes of NMDA receptors, of which the NR2B subtype appears to play a prominent role in these pathologic processes.1 The distribution of the NR2B subunit in specific forebrain regions (e.g., hippocampus) imparts a regional selectivity to CP-101,606 in brain areas that are especially vulnerable to injury from ischemia.1 Thus, NMDA receptor antagonists such as CP-101,606 that target NR2B subtype receptors may have therapeutic application in preventing the neurologic deficits and death caused by stroke. The primary objective of this study was to assess the ability of CP-101,606 to limit ischemic tissue volume growth following acute ischemic stroke by MRI measurement at 48 hours post-ictus. Exploratory statistical analyses were performed using a global test statistic that included a combination of clinical endpoints that once adjusted for baseline stroke severity and age2 noted a trend for efficacy associated with CP-101,606 therapy. The purpose of this pharmacokinetic-pharmacodynamic analysis was to evaluate the exposure-response relationship in this study and included a spectrum of covariates that are under investigation as predictors of clinical outcome following an ischemic stroke.
Study Design
Randomized, double-blind, placebo-controlled, parallel group study of CP-101,606 in subjects with an MRI confirmed acute ischemic stroke in the forebrain.CP-101,606 infused intravenously at 0.75 mg/kg/hr for 2 hours and 0.37 mg/kg/hr for 70 hours, or placebo within 8 hours of stroke onset.Non-compartmental PK parameters: AUC(0-Tlast), Cmax, Tmax, Css,avg and CLp.CYP2D6 genotyping was performed using standard polymerase chain reaction methodologies (EM=extensive metabolizer, IM=intermediate metabolizer, PM=poor metabolizer).
IRB approval and ICH Guidelines for GCP and the Declaration of Helsinki were followed.
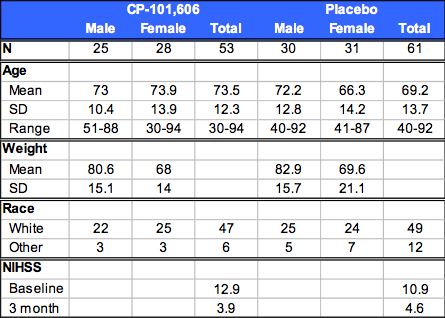
Table 1: Subject Demographics
Results
Figure 1. Individual Plasma Concentration vs. Time Profiles
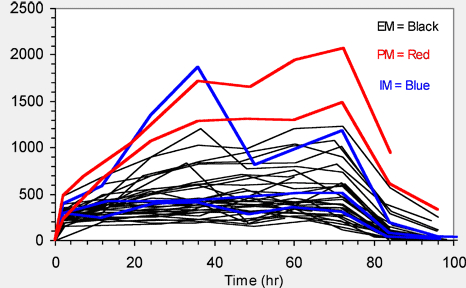
Table 2. Mean (SD) Non-CompartmentalL Pharmacokinetic Parameters
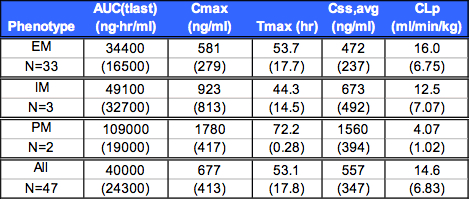
Summary of Non-Compartmental Pharmacokinetic Results
Css, avg 200 ng/ml for all subjects.
EM Css, avg approximately 3-fold less than PM.
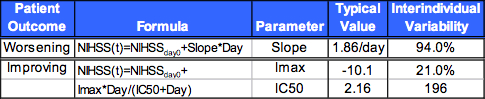
Table 4. Population Pharmacodynamic Modeling
Analysis of 1030 NIH Stroke Scores (NIHSS) from 114 patients
NIHSS observations modeled as two groups using a mixture model:
Patients that improved: nonlinear IMAX model.
Patients that worsened: linear model.
Covariates (sex, race, age, weight, BSA, infarct volume, baseline NIHSS, prior statin and ACE inhibitor treatment) were evaluated using the same method and criteria described for PK.
Using final PK model, exposure (AUC, Css,avg) and dose (active or placebo treatment, total CP-101,606 dose and exposure) were evaluated. Effect of CP-101,606 described as a linear function.
Higher baseline NIHSS was associated with poorer recovery (lower Imax).
Older age and larger lesion volume lengthened recovery time.
Higher CP-101,606 Css,avg was associated with greater recovery.
The likelihood ratio test indicated the statistical significance of the drug effect was somewhat low (p<0.05); however, addition of drug effect significantly decreased the variability of the parameters.
Figure 6. Simulated Stroke Sscores Ffor Different CSS, Avg Concentrations
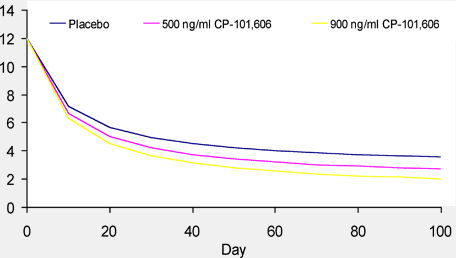
Conclusions
The dosing regimen administered in this study resulted in stoke patients achieving or exceeding the targeted minimum Css,avg of 200 ng/ml.
CYP2D6 extensive metabolizer genotype patients had ~66% lower exposure than poor metabolizers.
A mixture model for assignment of EM/PM status resulted in a larger proportion (~27%) of slow metabolizers than observed in the general population (~15%), and by genotyping (~10%), possibly due to the large variability of CYP2D6 expression in the population.
Higher CP-101,606 Css,avg was associated with greater recovery.The population PK/PD modeling results were consistent with the statistical analysis for this study, which used a dichotomized NIHSS with adjustments for age and baseline NIHSS imbalances2, and suggested a benefit for CP-101,606-treated stroke patients.
Simulation of NIHSS using the PK/PD model developed here suggests greater exposure to CP-101,606 results in a more complete recovery (lower NIHSS).
Further study of CP-101,606 therapy to evaluate clinical efficacy in acute stroke is warranted.